
table of contents
- Why is a pH that is too high dangerous?
- pH test strips
- How big is my swimming pool?
- Lowering the pH
- How do you proceed?
- frequently asked Questions
In summer, the domestic swimming pool offers a real alternative to overcrowded outdoor pools. But that only applies if the water quality is right. These measures help to lower the pH level.
In a nutshell
- Even small deviations in the pH value have serious effects on technology, bathers and the need for chemicals
- any acid is able to lower the value
- An exact calculation of the tank volume and acid requirement is essential to achieve the target value
Why is a pH that is too high dangerous?
Depending on the pH value, water is either acidic, neutral or basic. A value between 7.0 and 7.4 is usually ideal for the water in your pool, because then the water quality is neutral to minimally alkaline. If, on the other hand, the value rises above this limit, one speaks of a so-called alkalosis. Up to 7.5 it is still a slight alkalosis. In all other values, however, it is a matter of severe alkalosis, which must be assessed critically from various points of view:

- Reduced effectiveness of the Chlorine
- As a result: rapid formation of algae and multiplication of microorganisms
- Damage to human skin through dehydration and degreasing
- In the long run, further health risks due to impaired oxygen uptake of the blood (blood pH value around 7.4), resulting in dizziness and nausea
- Increase corrosion on metal components (rust)
- Overall increased load on all components of the pool
Note: Depending on the pool size and structure, the ideal value for the water quality can vary slightly within the framework shown. Let the manufacturer advise you on the optimal pH value for you when purchasing the pool.
pH test strips
How do you even measure the value of water quality? There are various technical possibilities for this. So-called pH test strips are most common in the private sector. Briefly hold the strips in the water of your pool and wait for the indicator paper to change color. You can then read the exact pH value from the enclosed color table.
Note: The evaluation of the pH value must always be carried out with the associated reference cards! If you use the color card of other test strips, the colors may vary and falsify your result!

If you suspect that your pH value is too high, it is essential that you always take an accurate measurement before taking any further steps. Because various factors can cause the value to change within a few hours:
- Water temperature
- Exposure to sunlight
- Other substances in the pool water (pool chemicals, dirt, etc.)
- Use by bathers
Then it can happen that you want to act, but your efforts are going in the wrong direction or even no longer necessary.
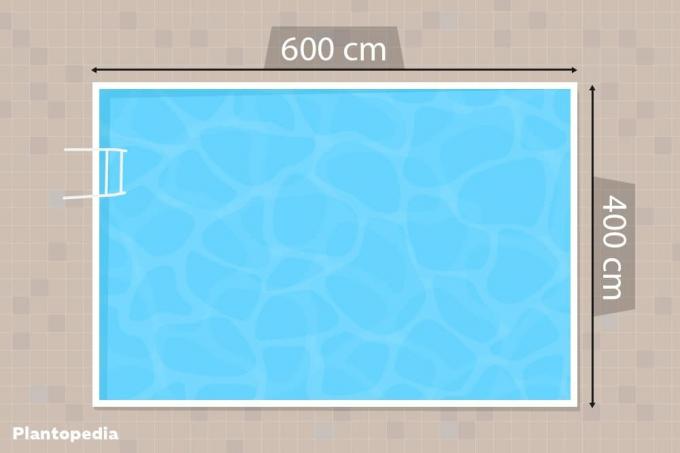
How big is my swimming pool?
No matter how you want to bring your pool water back into balance, the most important parameter for all measures is the water volume. Because the required total amount of water additives always results from a specific requirement per cubic meter of pool volume. This is how you can easily determine the amount of water in your pool:
Rectangular floor plans:
- Length x width x water depth (in meters) = water volume in cubic meters
- For example: 4 meters wide, 7 meters long, 1.20 meters deep
- 4.00m x 6.00m x 1.20m = 28.80 m3
Round floor plans:
- Base area according to the circular formula Pi x radius in the square x water depth (in meters) = water volume in cubic meters
- For example: 4 meters in diameter, 1.20 meters deep
- 3.14 x (2.00m) ² x 1.20m = 15.07 m3
Tip: You can also easily calculate a pool with a different floor plan. Simply subdivide the base area into approximately matching rectangles and circular segments. You can then calculate and add up these as described.

Lowering the pH
If the value of your pool water is too high, all you have to do is use chemicals to get back into the acceptable, medium pH range. There are several options available to you for this:
pH reducers
Many pool accessories suppliers offer special pH reducers. These come either as granules or as an aqueous solution and are added to the water. These special agents usually contain various inorganic acids, such as:
- phosphoric acid
- hydrochloric acid
- Other acids
Depending on the product and manufacturer, the acid content and thus also the requirement can vary depending on the desired pH reduction per cubic meter of water. It is essential that you observe the dosage instructions as well as general hazard and usage instructions for handling the products.
vinegar
If you want to do without expensive pool chemicals from specialist retailers, you can also use other, usually much cheaper acids. Vinegar, for example, is well suited. Vinegar is also an organic acid and can therefore be used without hesitation. Make sure, however, that it is pure vinegar essence, as the following often found additions are culinary exciting, but only have disadvantages for your pool:

- Sugar (e.g. B. in balsamic vinegar) - sugar promotes germ growth
- Unfiltered natural vinegars - cloudy substances etc. as a breeding ground for bacteria
- Other flavors and additives
citric acid
Pure citric acid from household needs is also wonderfully suitable for reducing the water characteristics of your pool. The advantage of this substance, which is mostly used for containment, is its high purity and the absence of disadvantageous additions. Citric acid is usually present as a crystalline powder that can simply be added to the water.

hydrochloric acid
Theoretically, the use of low-dose hydrochloric acid would also be possible. However, you should only use hydrochloric acid if you have the necessary knowledge about handling the hazardous substance. If used improperly, there is a high risk of chemical burns and permanent damage to the lungs or eyes.
Note: If you decide on home remedies that are not specially offered for the pool, the dosage will usually be less precise than is the case with special pH reducers. Slowly feel your way forward and test the result again and again. Otherwise, you can easily overshoot your target and cause hyperacidity.
How do you proceed?
Used incorrectly, you can achieve very different, often negative effects in addition to "acidic" water with pH-lowering agents. Therefore, make sure to use the application correctly.
the initial situation
Since changes in temperature, the amount of water, the pool chemistry used, but also possible cleaning processes have a strong influence on the Characteristic of the water, you should make the adjustment of the value under conditions that, if possible, the later bathing operation correspond. The afternoon on the day before the bathing is open, for example, is therefore well suited. The late evening or early morning hours are rather poorly suited, as the water temperature and sun exposure then differ significantly from the times of use.

The sequence
If the value is too high, you can reduce it step by step with these measures:
- Put the pumping system into operation for sufficient water movement
- Pour pH reducer freely into the pool water
- Keep the circulation in operation for around 10 minutes to ensure that the water in the entire pool is equalized
- Measure the pH value using a test strip
- add further pH reducers if necessary
Attention: Never put the acids directly into the skimmer, the filter system or other technical components. Otherwise there will be an extreme drop in pH that can damage sensitive components in a short time.
frequently asked Questions
The search for the cause is heavily dependent on your individual circumstances. A likely cause is regular post-chlorination. Due to its chemical properties, chlorine is able to change the pH value upwards.
Ultimately, you can use any acid regardless of its concentration. However, weaker acids must be added in larger quantities than stronger acids.
Technical components can still cope with minor deviations quite well. The human organism in particular reacts very sensitively, even with small deviations. The effectiveness of the chlorine also decreases significantly even with small deviations. The result would be reduced water purity or a significantly higher chlorine content.
